Our Speciality Includes
Pharmaceutical HVAC is one of the most crucial utility for any type of facility, be it OSD, Injectable, Hormone, MDI, Cytotoxic, and so on. Moreover, the energy consumption by HVAC alone is around 65% of the total plant. So, it requires engineers with sound technical expertise and experience to not only deal with these facts but to help you guide through the current regulatory trends and demands, be it, USFDA, MHRA, WHO, PIC/S, TGA MHLW , the ISO standards, or the recent Pharma 4.0 initiative.
The regulatory requirements within the pharmaceutical industry are becoming increasingly stringent. The GMP regulations are subject to constant development, although clear instructions are rarely issued. And to make matter even worse every country has their own regulatory body with each regulatory having its own set of rules so harmonization of these regulatory bodies is a task. Nevertheless, your investment has to be fit for the future, so you can be rest assured that there will be no flaws on the regulatory front. This requires a careful analysis of the demands made by authorities and a reliable interpretation of the GMP regulations with a Risk Assessment to adhering to the compliance.
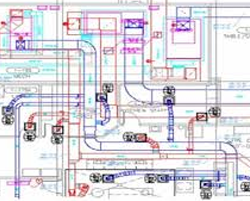

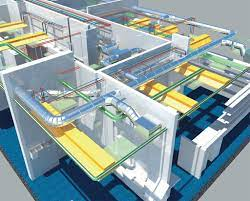
The pharmaceutical industry is undergoing rapid changes, competition will increase continuously, while pressure of costs on individual companies will continue to rise. This requires the development of more efficient solutions, with new paths being trod in the process. Current projects have to be executed within ever shorter time limits and are subject to strictly predetermined budgets. Meanwhile, The technology thereby employed has become increasingly complex and demanding.
The key to sound execution is coordination not only with the consultants but other contractors as well. An optimum flow of information between the disciplines is crucial to a project's success or failure. A team of specialists with interdisciplinary knowledge is required in order to work together to interlink these elements while ensuring safety, efficacy, and efficiency.



After installation and cleaning, the clean rooms will be subject to extensive tests. Our qualification and validation team will provide and support you with the necessary information on complying with the room's conditions in terms of ventilation, differential pressure, temperature, humidity, particles and microorganisms, smoke studies, temperature mapping, where required, during qualification.
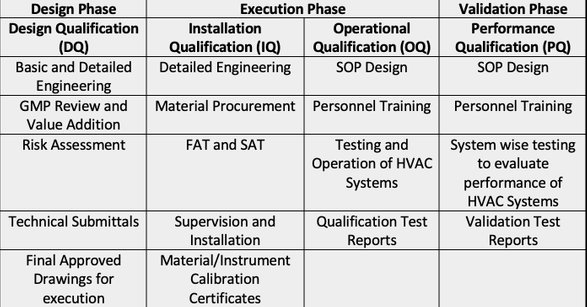
We put a lot of effort and focus on documentation as they are the nuts and bolts of the entire plant. The efficient coordination of acceptance tests, commissioning activities and qualification is our top priority. This integrated process helps us to avoid double work and allows us to qualify the facilities quickly and efficiently. If any discrepancies arise, these are documented and dealt with "change control system".
24*7 Customer Care Support No: 9824317840 or Mail Us : info@machvac.in